On average, over 1.7 million community-based COVID-19 tests are conducted each day. And for a patient, it’s a two-step process. First, a healthcare professional extracts a tissue sample and then in a few days the patient receives the test result. If it's positive, they start a treatment regimen. But, what patients don’t see are the complex steps in between and how those steps sometimes impede the speed and accuracy of test results.
Because of the number of tests, the inability to automate certain LIS system data entry steps, poorly or mislabeled specimens and test result communications, it can cause testing outcome delays and even a need for retesting. Plus, with the announcement of a 90% successful vaccine during interim analysis from Pfizer/Biontech and Moderna, it highlights another challenge: inoculating millions of citizens throughout the world. And although overcoming these problems isn’t easy, there are steps you can take to improve the process. In fact, these 5 solutions to lab testing bottlenecks relating to COVID-19 will improve the speed and accuracy of testing and mitigate potential challenges with vaccine administration.
5 Solutions To Lab Testing Bottlenecks Caused By COVID-19
The potential for process improvements center around these major chokepoints:
- Specimen Collection
- Specimen Logistics
- Specimen Testing
- Test Result Communications
And once the COVID-19 vaccine is available:
- Vaccination Procedures
But you can improve workflows by implementing these 5 solutions to lab testing bottlenecks caused by COVID-19.
Specimen Collection Bottlenecks Caused By COVID-19
Take a trip back in the time machine all the way to 2019. You visited your doctor with a symptom that required a specimen for an accurate diagnosis. To obtain the sample, your doctor had a nurse or another healthcare professional, most likely in a primary care facility or commercial lab, collect it.
But with COVID-19, that specimen collection process is no longer standard operating procedure. For example, testing takes place in numerous disparate settings ranging from the controlled environment of a primary care facility to the variable conditions of a field testing center. And although healthcare professionals are sometimes involved in the process others, including the patient themselves, may also perform that task.
Next, once the tissue sample is extracted, it’s placed into a sterile container and labeled with patient identification information. Just like the other steps in the specimen collection process, the people responsible for this step vary. It could be a healthcare professional or even a volunteer with no previous experience in specimen collection.
The label placed on the container includes patient identification information that ensures the test results get analyzed properly and communicated back to the patient in a timely manner. Yet, when you marry these diverse processes with the exponential increase in tests processed, it creates additional problems.
Solutions To Specimen Collection Bottlenecks Caused By COVID-19
 Since each specimen ties back to the patient, accurate identification is essential. Regardless of the size of the specimen collection device, a small vial or slightly larger cup, the information must include at least two patient-specific identifiers and many specimen identifiers including:
Since each specimen ties back to the patient, accurate identification is essential. Regardless of the size of the specimen collection device, a small vial or slightly larger cup, the information must include at least two patient-specific identifiers and many specimen identifiers including:
- Patient Name
- CDC Number
- Unique Random Number
- Medical Record Number
- Medicaid Number
- Newborn Screening Kit Number
- Date of Birth
Further, this information is contained on a label that’s applied to the specimen. Unfortunately, numerous complications can disrupt this process. First, key patient information is sometimes missing. If so, the sample is rejected and the patient is forced to be retested. Second, although some of the required information is often represented within a barcode, it requires the compression of many data points onto a small label that fits on the collection device. Plus, the small size and tight mandrel of the vial can cause additional labeling problems. Further, the high volume of tests combined with the variable weather conditions of a field testing facility, creates additional challenges. It can cause labeling difficulties ranging from:
- Misplaced labels on vials
- Labels only partially adhering to the vial
- Patient identification information detaching completely from the vial
Each issue can cause the sample to be rejected and force a retesting of the patient.
Lastly, although COVID-19 supply chain challenges are most often associated with the lack of PPE, swabs and other testing vehicles, LIS system labels are an essential supply chain component. They are vital to the specimen collection process.
Take These Steps To Ensure Specimen Labeling Accuracy
Throughout the testing process, COVID-19 specimen labels must remain attached to the specimen. That may sound simple but it’s not, because the label must withstand these requirements:
- Remain flexible to fit around a small diameter vial without curling or popping
- Avoid sticking to examination gloves
- Withstand temperatures as low as 0℃
- Resist laboratory solvents such as xylene, dimethyl sulfoxide and isopropyl alcohol - without curling or defacing
- Withstand the polymerase chain reaction (PCR) process that determines whether the sample is positive or negative - without curling or defacing
- Retain the printed barcode and patient identification information without smearing
Also, if vials are staged outside on a cold day, labels that meet freezer application temperatures may be required. Doing so will ensure the adhesive sets, sticks to the vial and doesn’t inadvertently pop off.
When you obtain LIS system labels, make sure they meet these specifications.
Specimen Logistics Bottlenecks Caused By COVID-19
Once COVID-19 specimens are collected, routing them to a lab testing facility is the next challenge. But given the wide range of testing facilities drawing specimens, this step creates additional complications.
First, specimens must be stored at 2 to -8°C for up to 72 hours after collection. And then ideally, shipped overnight on ice packs to the lab testing facility. But, if the collection and shipment process takes longer than 72 hours, specimens must be kept at-70°C or below and shipped on dry ice.
Solutions To Specimen Logistics Bottlenecks Caused By COVID-19
But when specimens require cold storage temperature shipment, other problems may emerge. For example, the extreme cold temperatures alter the label adhesive chemistry and can cause the patient identification label to detach from the vials. If a specimen arrives without this label, it gets rejected. Fortunately, cold temperature adhesives prevent this problem. So when procuring the LIS system labels, make sure they meet these specifications.
Additionally, when a label does fail, sometimes there is an effort to relabel a sample. Yet, during the pandemic, as in ordinary times, relabeling specimens can create major risks and potential harm to the patient according to an article in the Archives of Pathology and Laboratory Medicine.
Lastly, samples need to be effectively and securely tracked in a HIPAA compliant fashion. Scanning the sample upon pickup, and then upon delivery to the laboratory, serve to ensure patient specimens are tracked throughout the chain of custody.
Specimen Testing Bottlenecks Caused By COVID-19
On November 21, daily COVID-19 tests topped 1.9 million, more than double the average just two months earlier. Although necessary to combat the virus, this increase in testing exerts enormous demands on labs to timely analyze and process the tests. As a result, common clinical lab providers have increased capacity and organizations that previously didn’t provide this type of lab testing are now doing so. For example, Quest and LabCorp, two of the largest commercial labs in the U.S., are ramping up for even greater demand and many labs that didn’t previously conduct the molecular tests that now represent a high percentage of COVID tests, are processing hundreds each day.
Although the additional testing capacity is essential in order to meet demands, the huge number of tests create strains at multiple points through the process. The two most challenging areas are taking in samples and reporting results.
Solutions To Specimen Testing Bottlenecks Caused By COVID-19

When COVID-19 specimens arrive at a hospital, commercial, private or academic lab, the first challenge is entering data into a Laboratory Information System (LIS). Typically that process isn’t a challenge, but for COVID-19 samples, it is.
Because SARS-CoV-2 testing occurs in a variety of environments outside traditional healthcare settings that labs don't typically interact with, it makes interfacing with a LIS system problematic.
First, many LIS systems are not made to effectively onboard large groups of patients. Plus, even when digital processes are in place, it doesn’t always solve the problem. Philippe Flamant, vice president of solutions engineering at Elmwood Park, New Jersey-based healthcare data firm Ellkay said,
”Even labs with fully electronic accessioning systems run into problems if those systems aren’t tailored to the higher-throughput demands of SARS-CoV-2 testing.”
Consider a laboratory built for complex testing processes, like whole-genome sequencing, that have taken on COVID-19 testing. The LIS system asks the same very detailed genomic testing questions needed for COVID. But for COVID samples, those questions aren’t necessary. Unfortunately, this significantly increases data entry time with only one short-term solution, throwing more people at the problem.
In addition to this processing time increase, other issues slow the lab testing process. For instance, the specimen label is a key driver of patient information. But if the label is compromised when the sample arrives at the lab testing facility, the lab may not be able to process the sample.
To demonstrate, when a specimen is staged for analysis, the diagnostic system places it onto a conveyor where the vial passes through a barcode reader to record information about each sample. If the label is applied incorrectly, blocks specimen information, or detaches completely during the collection or logistics steps preventing a proper barcode reading, the system rejects it. Unfortunately, this added processing time is only one part of the problem. Redrawing the specimen is the other.
Communicating Test Results Bottlenecks Caused By COVID-19
Anxious is one word to describe most patients waiting for the outcome of their COVID-19 test. And the testing problems described above can cause delays, adding to a patient's stress level. But it’s not just testing that causes delays, communication is also a problem.
To illustrate, data from a Duke-Margolis study showed that up to 50 percent of COVID-19 laboratory reports did not contain the patient’s address or ZIP code. As a result, test results weren’t always communicated either to the patient or their primary care physician. In fact, they may never even know their patients were tested, making it unlikely that patient information will be matched to the right medical records. This missing communication link will also prevent healthcare providers from gaining a comprehensive view of the patient’s health history further down the line.
Similarly, when patients suspected they had the coronavirus and used telehealth services, related problems surfaced.
Privacy
Another communication challenge is maintaining an appropriate balance between serving public health and adhering to appropriate privacy levels. Health care providers are sending this information to public health departments and governments as they try to control the spread of the disease. Although it provides them with data to inform decisions about how to safely manage their communities, it’s essential to manage the data properly.
Solutions To Test Results Communication Bottlenecks Caused By COVID-19
Preventing the highest percentage of communication errors requires revisiting the specimen testing process. It’s imperative that patient data collection is thorough and the labeling process is executed properly.
Using barcodes, when possible, during specimen collection also guards against some privacy challenges. In addition, the data collected and maintained should be reviewed according to the following guidelines:
- Are data minimization principles being followed?
- What is the retention plan?
- Is there transparency in the process?
- Are there specific use limitations?
- How secure is the data collected?
COVID-19 Vaccine Bottlenecks
 On the heels of successful phase three clinical trials, both Pfizer and Moderna announced their COVID-19 vaccines will be available in the next 30 to 60 days. Plus, AstraZeneca is on the cusp of approval. And as a result, healthcare providers will soon add administering vaccinations to their other COVID-19 challenges.
On the heels of successful phase three clinical trials, both Pfizer and Moderna announced their COVID-19 vaccines will be available in the next 30 to 60 days. Plus, AstraZeneca is on the cusp of approval. And as a result, healthcare providers will soon add administering vaccinations to their other COVID-19 challenges.
But unlike the flu, shingles and other more common immunizations, the COVID vaccine requires cold storage initially, at nearly -100 degrees Fahrenheit, and then refrigeration to remain effective. Although the Moderna vaccine can be refrigerated for up to 30 days and remain effective, at this point, the Pfizer version remains viable for only five days. Managing the cold temperature demands to maintain the vaccines’ efficacy presents a major challenge.
In fact, Dr. Georges C. Benjamin, executive director of the American Public Health Association said, ”The big issue is not how to move vaccines across the country by major shippers. It is how to move it from them to pharmacies, retail clinics, doctors offices, etc., and maintain the ultra-cold environment.” Further, both the Pfizer and Moderna vaccines require two injections 21 to 28 days apart to achieve the 90%+ effectiveness reported in clinical trials. Ensuring patients return for the follow-up appointment is crucial to inoculation.
Solutions To Vaccine Bottlenecks Caused By COVID-19
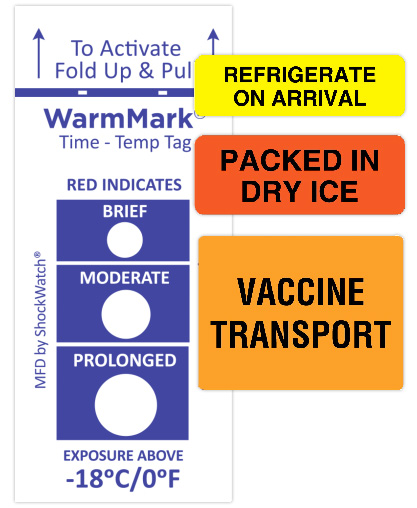 Manufacturing and distributing hundreds of millions of any product is challenging. But the complexity is far greater for the COVID-19 vaccine with the cold temperature requirements. Vaccines must be maintained within a narrow temperature range as they go from factories to shipping facilities to trucks to hospitals, clinics, and pharmacies, and eventually, into the arms of people. Although larger hospitals may have proper storage facilities, physicians’ offices and clinics typically do not. Yet cold storage controls are essential because every breach degrades the vaccine, making it less effective or ineffective altogether.
Manufacturing and distributing hundreds of millions of any product is challenging. But the complexity is far greater for the COVID-19 vaccine with the cold temperature requirements. Vaccines must be maintained within a narrow temperature range as they go from factories to shipping facilities to trucks to hospitals, clinics, and pharmacies, and eventually, into the arms of people. Although larger hospitals may have proper storage facilities, physicians’ offices and clinics typically do not. Yet cold storage controls are essential because every breach degrades the vaccine, making it less effective or ineffective altogether.
Using an ascending time-temperature indicator label is one way to ensure that vaccines were maintained at the correct temperatures. They provide an alert as to when exposure to an unacceptable temperature occurred and provide irreversible evidence of how long the exposure lasted.
In addition to cold storage, there are additional challenges. Each inoculation event is separated by 21 to 28 days. Reminding patients and ensuring they obtain the second shot is critical to optimal vaccine effectiveness. In fact, COVID-19 vaccination providers should make every attempt to schedule a patient’s second-dose appointment when they get their first dose. Although it’s inevitable that some patients will miss their follow up appointments, implementing a reminder process will increase compliance. Using a series of email, text, phone and physical reminders will maximize results.
Further, neither the Pfizer, Moderna, nor other vaccines in the pipeline, are interchangeable. That means the patient’s second dose must come from the same manufacturer as their first dose. Patient information will be recorded by the provider in an EHR system for some patients, making it clear which vaccine to use for the booster shot. But, with hundreds of millions of doses administered throughout the U.S., defining the vaccine type will present a challenge. Communicating the specific vaccine type with the follow-up appointment information will minimize confusion.
United Ad Label COVID-19 Lab Testing Solutions
Hopefully, the Pfizer and Moderna vaccines are the beginning of the end of the pandemic. But, there are many more months before we can say it’s over. In the meantime, with infection rates still increasing, these 5 solutions to lab testing bottlenecks caused by COVID-19 will increase the speed and accuracy of lab testing and mitigate potential challenges with vaccine administration.
